-
 Lithification
Lithification
-
 Myelomeningocoele
Myelomeningocoele
-
 Rodent
Rodent
-
 Radiation pressure
Radiation pressure
-
 Analgesic
Analgesic
-
 Capacitor
Capacitor
-
 Neurotransmitter
Neurotransmitter
-
 Genotype
Genotype
-
 Elementary particle physics
Elementary particle physics
-
 Deoxyribose
Deoxyribose
-
 STEREO
STEREO
-
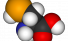 Selenocysteine
Selenocysteine
-
 Receptor
Receptor
-
 Polluter pays principle
Polluter pays principle
-
 Session key
Session key
-
 Extrasolar
Extrasolar
-
 Accommodation
Accommodation
-
 Isotropic
Isotropic
-
 Controlled fusion
Controlled fusion
-
 Laryngectomy
Laryngectomy
-
 Noma
Noma
-
 VLAN
VLAN
-
 Downy oak
Downy oak
-
 Curettage
Curettage
-
 Wheel motor
Wheel motor
-
 Propagule
Propagule
-
 Epicondyle
Epicondyle
-
 Bone marrow
Bone marrow
-
 Magnesium
Magnesium
-
 Nucleic acids
Nucleic acids
Tungsten
Tungsten is an element (symbol W), of atomic number 74, atomic mass 183.85, belonging to the third series of transition elements. A grey metal of density 19.3 g cm-3, melting point 3410°C (the highest of any metal) and boiling point 5660°C.
Its commonest oxidation state is 6.
Its ores are tungstates such as wolframite, scheelite, stolzite (PbWO4).
Tungsten is obtained by reduction of its oxide, which itself is produced by prior transformation of the ore (fusion in caustic soda).
It is refractory and is used to make arc furnace electrodes and incandescent lamp filaments. Tungsten is used in alloys, especially steels.
Tungsten alloys have been used since the end of the 19th century. Tungsten filaments were introduced by Auer and Coolidge. The evaporation of tungsten, which plays an important role in halogen lamps, was studied by Langmuir. Tungsten carbide tools go back to the 1920s.
Latest
Fill out my online form.



