-
 Marpol Convention
Marpol Convention
-
 POP3
POP3
-
 Electrical resistance
Electrical resistance
-
 Allele
Allele
-
 Conception
Conception
-
 SACD
SACD
-
 Carnivore
Carnivore
-
 DBMS
DBMS
-
 Browser hijackers
Browser hijackers
-
 M95
M95
-
 Prostrate shrub
Prostrate shrub
-
 Meson
Meson
-
 Brain death
Brain death
-
 Cepheid
Cepheid
-
 Norite
Norite
-
 Aspiration
Aspiration
-
 Replicase
Replicase
-
 Radish
Radish
-
 Dynamic super-resolution microscope
Dynamic super-resolution microscope
-
 Genotoxic
Genotoxic
-
 Crystallisation force
Crystallisation force
-
 Palm
Palm
-
 Metallicity
Metallicity
-
 Holoside
Holoside
-
 Phase diagram
Phase diagram
-
 Chemical dam
Chemical dam
-
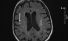 Leukoaraiosis
Leukoaraiosis
-
 Biocenosis
Biocenosis
-
 Orthosilicate
Orthosilicate
-
 The Bohr magneton
The Bohr magneton
Thermodynamics
Thermodynamics is the branch of physics and chemistry related to the thermal behaviour of bodies, the study of energy and its transformations (in particular, internal energy).
Thermodynamics. Thermodynamics is the study of the transformations of systems (collections of bodies that may or may not be separated by a material barrier), open or closed (according to whether or not matter is exchanged with the exterior), isolated or not (according to whether or not energy is exchanged with the exterior) represented by variables of state (intensive or extensive).
The basic theoretical notions of thermodynamics are heat, thermodynamic temperature, internal energy, enthalpy, entropy and reversibility.
The experimental values are heat capacities, pressure, volume etc.
The main laws of thermodynamics are the first principle, the second principle (V. Carnot, Clausius) and the third principle (V. Nernst) of thermodynamics.
The study of the thermodynamics of bodies includes the design and validation of models for the thermal behaviour of bodies, equations of state, established using experimental values. In practice, the equilibria predicted by thermodynamics can be contradicted by the influence of time (reaching thermodynamic equilibrium can sometimes take an infinite time) and are governed by kinetics (cf. Arrhenius). In chemistry, thermodynamic equilibrium is governed by the law of mass action (V. Guldberg).
Latest
Fill out my online form.



