-
 Graph theory
Graph theory
-
 Phoenicopteriformes
Phoenicopteriformes
-
 Adventitious
Adventitious
-
 RADARSAT-1
RADARSAT-1
-
 Green sea turtle
Green sea turtle
-
 Hem
Hem
-
 Cardiopathy
Cardiopathy
-
 Absolute density
Absolute density
-
 Smallpox virus
Smallpox virus
-
 Morphology
Morphology
-
 Bell pepper
Bell pepper
-
 Cellulite
Cellulite
-
 Dabbling duck
Dabbling duck
-
 Chloracne
Chloracne
-
 Craton
Craton
-
 Contraceptive implant
Contraceptive implant
-
 Aromatherapy
Aromatherapy
-
 Ganglion
Ganglion
-
 Fair trade
Fair trade
-
 Instant messaging
Instant messaging
-
 Vacuum
Vacuum
-
 Browser
Browser
-
 UNEP
UNEP
-
 Nix
Nix
-
 Bosporos planum
Bosporos planum
-
 Arianespace
Arianespace
-
 Alar bar
Alar bar
-
 Hypothesis
Hypothesis
-
 Nuptial plumage
Nuptial plumage
-
 Cinnabar
Cinnabar
Supercritical fluid
Supercritical fluids are produced by heating a gas to above its critical temperature or by compressing a liquid beyond its critical pressure. The critical temperature of a substance is the temperature beyond which a liquid phase cannot exist, whatever the pressure. The critical pressure of a substance at its critical temperature is its vapour pressure. When a substance is at temperatures and pressures beyond but still close to its critical temperature and pressure (the critical point), the substance is called a supercritical fluid.
Under these conditions the molar volume is the same, whether the original form was liquid or a gas. Supercritical fluids have densities, viscosities and other properties that are intermediate between those of the gas state and those of the liquid state of the substance. Carbon dioxide is the most commonly used supercritical fluid because of its low critical temperature (31°C), its inert properties, its low toxicity and reactivity plus its high purity and low cost. Carbon dioxide does not dissolve polar compounds, and so during the analysis of this type of compound, methanol, cyclic ethers, water or formic acid can be added to the carbon dioxide.
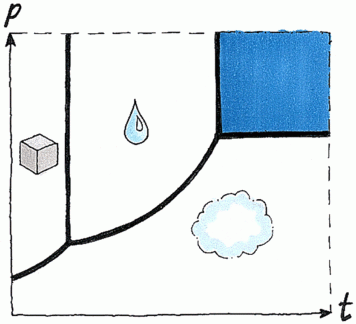 Phase diagram of carbon dioxide.
Phase diagram of carbon dioxide.
Latest
Fill out my online form.



