-
 Xenotransplant
Xenotransplant
-
 Emission spectrometry
Emission spectrometry
-
 JAXA
JAXA
-
 ATPase
ATPase
-
 Gravitational assist
Gravitational assist
-
 Sunspot
Sunspot
-
 Solar panel
Solar panel
-
 OCO
OCO
-
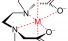 Chelation
Chelation
-
 Flamsteed number
Flamsteed number
-
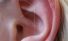 Middle ear
Middle ear
-
 Amnesia
Amnesia
-
 Genetic polymorphism
Genetic polymorphism
-
 ETSI
ETSI
-
 Western red cedar
Western red cedar
-
 Eruption
Eruption
-
 Exothermic
Exothermic
-
 Fosfomycin
Fosfomycin
-
 Gauge theory
Gauge theory
-
 Virgo
Virgo
-
 Betavoltaic
Betavoltaic
-
 Ctenophore
Ctenophore
-
 Optical isomerism
Optical isomerism
-
 Proline
Proline
-
 Thrust
Thrust
-
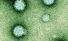 Chikungunya virus
Chikungunya virus
-
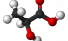 Lactate
Lactate
-
 Osterrite
Osterrite
-
 Kinetic energy
Kinetic energy
-
 Alar
Alar
Stimulated emission
The classical description of stimulated emission is that an electron can absorb a photon (with a probability associated with the Einstein absorption coefficient). One of two things may then happen:
Either the electron spontaneously and isotropically re-emits a photon. This is referred to as spontaneous emission which occurs with a certain probability associated with a time constant. Since the electron is free (not bound to an atom), the absorption of the photon is coupled to an energy continuum in the electron's phase space. As a result the electron may re-emit a photon in a different state from the incident photon.
Or the electron collides with another photon causing the emission of twin photons This is referred to as stimulated emission and is the hypothesis that needs to be tested. According to the Einstein coefficients, this re-emission should be significantly preponderant over spontaneous emission.
Latest
Fill out my online form.



