-
 SST
SST
-
 Umbriel
Umbriel
-
 Dyslexia
Dyslexia
-
 Ovigerous
Ovigerous
-
 Type III star population
Type III star population
-
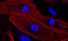 Cardiac myocyte
Cardiac myocyte
-
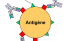 Antibodies
Antibodies
-
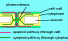 Symplast
Symplast
-
 Immunomodulator
Immunomodulator
-
 Bituminous shale
Bituminous shale
-
 Radiation pressure
Radiation pressure
-
 Ares I
Ares I
-
 Calipso
Calipso
-
 Broccoli
Broccoli
-
 Apastron
Apastron
-
 systemic antifungals
systemic antifungals
-
 Photolyase
Photolyase
-
 Germinal layer
Germinal layer
-
 SAFER
SAFER
-
 Selecting
Selecting
-
 Plant litter
Plant litter
-
 Coppice
Coppice
-
 Gel-coat
Gel-coat
-
 Psycho-oncologist
Psycho-oncologist
-
 Artificial meadow
Artificial meadow
-
 Petiole
Petiole
-
 Host plant
Host plant
-
 Diphtheria
Diphtheria
-
 Phase angle
Phase angle
-
 Chromium
Chromium
NMR
Nuclear magnetic resonance (NMR) is based on the measurement of the absorption of radio frequency radiation (RF) by an atomic nucleus in a strong magnetic field.
The absorption of radiation makes the nuclear spin realign or turn in the direction of highest energy. After having absorbed energy the atomic nuclei re-emit RF radiation and return to their initial state of lowest energy level.
The NMR principle is as follows: atomic nuclei with an odd number of protons, neutrons or both will have an intrinsic nuclear spin. When an atomic nucleus with a non-zero spin is placed in a magnetic field, the nuclear spin can align itself either in the same direction as the field or in the opposite direction. These two types of spin alignment have different energies and applying a magnetic field helps lift the degeneracy of the nuclear spins. An atomic nucleus of which the spin is aligned with the field will have a lower energy than when the spin is aligned in the opposite direction to the field.
The energy of an NMR transition depends on the force of the magnetic field together with a proportionality factor applying to each nucleus called the gyromagnetic ratio. The local environment around a given nucleus in a molecule has a tendency to slightly disturb the local magnetic field exerted on this nucleus and to affect its exact transition energy. This dependence of the transition energy on the position of a particular atom in a molecule makes NMR extremely useful in determining the structure of molecules.
NMR spectroscopy is one of the most powerful instruments for the determination of the structure of both organic and inorganic species. The technique has also proved useful in the quantitative determination of absorbing species.
Latest
Fill out my online form.



