-
 Tectrix
Tectrix
-
 Transmission electron microscope
Transmission electron microscope
-
 Toslink
Toslink
-
 Chondrite
Chondrite
-
 DDT
DDT
-
 Cathode
Cathode
-
 Biological corridor
Biological corridor
-
 Corot
Corot
-
 Jaundice
Jaundice
-
 Interference
Interference
-
 OSI Model
OSI Model
-
 Echocardiography
Echocardiography
-
 Antitussive
Antitussive
-
 Ester
Ester
-
 CMP
CMP
-
 Vitamin B9
Vitamin B9
-
 Thermoplastic
Thermoplastic
-
 Adware
Adware
-
 Miller indices
Miller indices
-
 Compton scattering
Compton scattering
-
 Sedge
Sedge
-
 Chronic pollution
Chronic pollution
-
 Premammalian
Premammalian
-
 Abnormality
Abnormality
-
 Long distance
Long distance
-
 TNM Classification
TNM Classification
-
 Radiant
Radiant
-
 Asthma
Asthma
-
 Anti-hormone
Anti-hormone
-
 Phenotype
Phenotype
Eutectic
Thermodynamics: A eutectic is a property of a solid mixture in specific proportions that has a constant melting point (eutectic point) like that of pure substances.
The most well known eutectic is salt+water; salting the roads in winter lowers the melting point of the mixture obtained. The melting point of a eutectic mixture is lower than those of its two components.
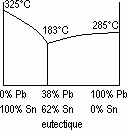
Eutectic: pure lead melts at 325°C, pure tin melts at 235°C and the mixture of 38% lead with 62% tin melts at 183°C. This mixture is called the eutectic and the temperature of 183°C is the eutectic temperature.
Latest
Fill out my online form.



