-
 Acrosome
Acrosome
-
 Severe persistent asthma
Severe persistent asthma
-
 Hydromorphy
Hydromorphy
-
 Capacitor
Capacitor
-
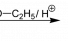 ETBE
ETBE
-
 Correspondence principle
Correspondence principle
-
 Lustre
Lustre
-
 Catechol-O-methyltransferase inhibitor
Catechol-O-methyltransferase inhibitor
-
 Air
Air
-
 Prophage
Prophage
-
 Panspermia
Panspermia
-
 Anemophily
Anemophily
-
 NRO
NRO
-
 Coma
Coma
-
 Bile duct
Bile duct
-
 Elasticity
Elasticity
-
 Deep convection
Deep convection
-
 Dolby Digital 5.1
Dolby Digital 5.1
-
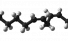 Linoleic acid
Linoleic acid
-
 Phase contrast microscope
Phase contrast microscope
-
 M32
M32
-
 Gray
Gray
-
 Botryoidal
Botryoidal
-
 VSC, PVC
VSC, PVC
-
 Breccia
Breccia
-
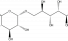 Isomalt
Isomalt
-
 Tensor
Tensor
-
 Organoleptic
Organoleptic
-
 Mitochondrial DNA
Mitochondrial DNA
-
 Phase transition
Phase transition
Extracellular matrix
The components of the bone matrix are formed to a large extent from type I collagen. Type I collagen is a fibrillar protein which is composed of three protein chains bound to each other fin a triple helix. It is produced by osteoblasts and forms sheets which interconnect (pyridine cross links) making the bone resistant to stretching. The organised structure is defective and the bone is fragile if bone is produced rapidly. Bone also contains small amounts of other collagen and non-collagen proteins (such as glycoproteins). Some such as osteocalcin are specific to the bone whereas others are not (osteopontin, fibronectin, and various growth factors). The function of these proteins has not been clearly defined, although they are assumed to be responsible for binding the bone cells to the matrix and for regulating bone activity. They also form a scaffold for the mineralisation of bone matrix that has not yet calcified (osteoid).
Mineralisation
The osteoid mineralises after 10 days. Hydroxyapatite crystals are deposited between the collagen fibres, making the bone resistant to breaking and stretch resistant.
Latest
Fill out my online form.



