-
 Tadpole
Tadpole
-
 PET
PET
-
 Pulsar
Pulsar
-
 Mean free path
Mean free path
-
 Metal
Metal
-
 Tropical forest
Tropical forest
-
 Group of galaxies
Group of galaxies
-
 Solvent
Solvent
-
 Hydrazine
Hydrazine
-
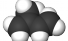 Isoprene
Isoprene
-
 Topographer
Topographer
-
 Intrauterine device
Intrauterine device
-
 Montrachet
Montrachet
-
 NTSC
NTSC
-
 Scanning electron lithography
Scanning electron lithography
-
 Diatomite
Diatomite
-
 Binary diagram
Binary diagram
-
 Antagonist
Antagonist
-
 Medical team
Medical team
-
 Pigmented
Pigmented
-
 Dual core
Dual core
-
 Carcinogen
Carcinogen
-
 RC2
RC2
-
 Transplant organ or tissue
Transplant organ or tissue
-
 Reduced substance
Reduced substance
-
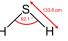 Hydrogen sulphide
Hydrogen sulphide
-
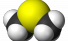 DMS
DMS
-
 VBR
VBR
-
 Chromatic aberration
Chromatic aberration
-
 Public domain software
Public domain software
Carbon
Carbon is a non-metallic chemical element (symbol C), of atomic number 6, atomic mass 12.01, belonging to group 14 in the periodic classification, and of valency 4.
The element has a melting point of 3550°C and a boiling point of 4827°C; it exists in the solid state in three crystalline forms: graphite, diamond and fullerenes.
Graphite, with the stacking of weakly inter-linked planes of carbon hexagons, is a good electrical conductor; diamond, a crystal with cubic symmetry in which each carbon atom is bound to four atoms at the vertices of a regular tetrahedron, is a good electrical insulator and a good conductor of heat; fullerenes are closed molecules (spheres, cylinders) formed from one or more sheets with a graphite structure with a fraction of the hexagons replaced by pentagons to accommodate the curvature.
There are also amorphous forms such as carbon black.
Carbon is a fuel and a reducing agent; it exists in the natural state, combined in hydrocarbons, in the form of oxides such as carbon dioxide, in carbonates (particularly calcium, as limestone and chalk), and in all organic molecules such as fats, carbohydrates (glucides or sugars) etc.
Latest
Fill out my online form.



