-
 SRS
SRS
-
 Spectroheliograph
Spectroheliograph
-
 Lead poisoning
Lead poisoning
-
 Accommodation
Accommodation
-
 Ocean planet
Ocean planet
-
 Laryngectomy
Laryngectomy
-
 Nutricline
Nutricline
-
 Gneiss
Gneiss
-
 One-time pad
One-time pad
-
 Autostereoscopy
Autostereoscopy
-
 Pollen
Pollen
-
 Contraceptive vaginal ring
Contraceptive vaginal ring
-
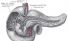 Exocrine gland
Exocrine gland
-
 Magma chamber
Magma chamber
-
 Genetic pollution
Genetic pollution
-
 Worm
Worm
-
 Celestial equator
Celestial equator
-
 Epileptic seizure
Epileptic seizure
-
 Tesla
Tesla
-
 Infusion
Infusion
-
 Helium
Helium
-
 AES/EBU
AES/EBU
-
 Magnetic moment
Magnetic moment
-
 Arianespace
Arianespace
-
 Cachexia
Cachexia
-
 Birds Directive
Birds Directive
-
 Stealth
Stealth
-
 Liquid laser
Liquid laser
-
 Ankylosis
Ankylosis
-
 Tau
Tau
Transition metal
The transition metals are the 30 chemical elements with the atomic number 21 to 30, 39 to 48, and 71 to 80. Their name comes from their position in the periodic table of elements, which represents the successive addition of one electron in the d orbital of the atoms on going from one element to the next across a period. The transition metals are chemically defined as "the elements that form at least one ion with a partially filled d sub-shell. "
Chemical properties
The transition elements generally have a high density and a high melting and boiling point. These properties are due to the ability of the electrons in the d shell to delocalise in the metal lattice. In metal substances the greater the number of electrons shared between the nuclei, the stronger the metal.
Here are four common properties of transition metals:
- they form coloured compounds;
- they have many oxidation states;
- they are good catalysts;
- they can form complexes.
Source: © Wikipedia
Latest
Fill out my online form.



