-
 Clinker
Clinker
-
 iDTV
iDTV
-
 Retified wood
Retified wood
-
 Primitive ocean
Primitive ocean
-
 Rigidity
Rigidity
-
 Testosterone
Testosterone
-
 Haemorrhoids
Haemorrhoids
-
 Atheroma
Atheroma
-
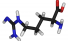 Arginine
Arginine
-
 Argilite
Argilite
-
 RC4
RC4
-
 Nuclease
Nuclease
-
 Goniometer
Goniometer
-
 Radon
Radon
-
 Bhopal disaster
Bhopal disaster
-
 Tidal Gate
Tidal Gate
-
 Aqua regia
Aqua regia
-
 Saline aquifer
Saline aquifer
-
 Avocado
Avocado
-
 M65
M65
-
 Restriction enzymes
Restriction enzymes
-
 Analogue
Analogue
-
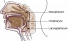 Pharynx
Pharynx
-
 BSC
BSC
-
 Proboscis
Proboscis
-
 Space-time diagram
Space-time diagram
-
 Duplex
Duplex
-
 El Niño
El Niño
-
 Doppler
Doppler
-
 Non-essential amino acids
Non-essential amino acids
Osmosis
Osmosis is the passage of solvent molecules, generally water, through a semi-permeable membrane from the medium least concentrated in solutes (hypotonic) towards the more concentrated medium (hypertonic).
The phenomenon ceases when the two liquids separated by the membrane reach the same concentration. This is referred to as an isotonic environment. The hydrostatic pressure due to the difference in water level between these two environments then compensates the osmotic pressure.
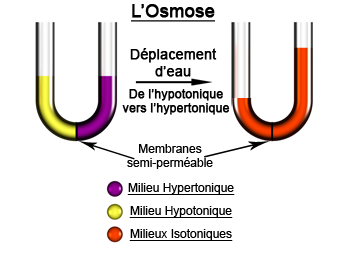 Illustration of experimental osmosis. © PsYcHoTiK CC by-sa
Illustration of experimental osmosis. © PsYcHoTiK CC by-sa
Latest
Fill out my online form.



