-
 Biogeography
Biogeography
-
 Peristome
Peristome
-
 Nucleon
Nucleon
-
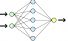 Neural network
Neural network
-
 PALM/STORM microscopy
PALM/STORM microscopy
-
 American space shuttle
American space shuttle
-
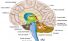 Brain
Brain
-
 Raman effect
Raman effect
-
 Gliding.
Gliding.
-
 Itching
Itching
-
 Meristem
Meristem
-
 Altimetry
Altimetry
-
 OSPAR Convention
OSPAR Convention
-
 Sub-critical reactor
Sub-critical reactor
-
 Axion
Axion
-
 Spiral galaxy
Spiral galaxy
-
 Akari
Akari
-
 Ferrofluid
Ferrofluid
-
 Electronic signature
Electronic signature
-
 Amalgam
Amalgam
-
 Emergence
Emergence
-
 Amine acid group
Amine acid group
-
 Premammalian
Premammalian
-
 Gauss constant
Gauss constant
-
 Amoeba
Amoeba
-
 Lahar
Lahar
-
 Iridescence
Iridescence
-
 Non-selective, monoamine oxidase inhibitor.
Non-selective, monoamine oxidase inhibitor.
-
 Spindle
Spindle
-
 Rate determining step
Rate determining step
Depleted uranium
After separation of the enriched fraction the remaining uranium contains about 99.8 % of 238U, 0.25 % of 235U and 0.001 of 234U. It has become what is called depleted uranium (DU).
DU mainly differs from natural uranium in its 235U content which is at least three times lower.
DU is therefore weakly radioactive and, for equal masses, it emits only 60% of the radiation of natural uranium.
Uranium and DU behave in the same way in the organism.
The uranium used in nuclear reactors is sometimes reprocessed in the enrichment facilities for natural uranium. It can therefore occur that radioisotopes created by these reactors contaminate the recycling material and as a result the DU.
It can, under these conditions, contain another isotope, 236U, and traces of the transuranium elements plutonium, americium and neptunium together with a fission product, technetium 99. However, based on the concentrations of these isotopes observed in DU, the increase in the radiation dose absorbed by the human organism does not exceed 1%.
Applications of depleted uranium
Its high density, about twice that of lead, has mainly led to the use of DU in the following civilian applications: counterweights in aircraft, protective shields in radiotherapy equipment and containers for transporting radioactive materials. The military also use it for defensive armour.
It is also its density, together with its property of catching fire if the temperature exceeds 600 °C at the point of impact, that makes it useful in armour piercing weaponry.
Exposure to uranium and depleted uranium
In most circumstances the use of DU is only an insignificant contribution to the intensity of natural uranium radioactivity in the environment. Conflicts in which DU ammunition is used probably have the highest risks of exposure.
A recent report from the United Nations Environment Programme (UNEP) gives the results of measurements made in the field around selected impact sites in Kosovo (former Federal Republic of Yugoslavia) and shows that contamination of the environment does not extend beyond a few dozen metres around these sites. The contamination of local vegetation and water resources by DU particles was found to be extremely low. The possibility of high exposure of local population is therefore considered to be very low.
In, November 2002 a team of United Nations experts announced that they had found traces of DU in three of the 14 sites studied in Bosnia following NATO air strikes in 1995. The UNEP published its complete report in March 2003.
Nevertheless, significant increases in concentrations of DU are sometimes observed near contaminating events. Normally, in the days and years following contamination it is dispersed into the environment by wind and rain, and people living or working in the affected areas can inhale particles or consume contaminated food or water.
People near to where a plane has recently crashed may be exposed to DU particles if the counterweights have been exposed to intense heat for long periods. But high exposure of this type should remain rare because there is little risk of the large masses of DU in the counterweights catching fire and they are slow to oxidise. Emergency and site clearance personnel may also be exposed but the normal professional protective measures should prevent any major exposure.
Latest
Fill out my online form.



