-
 Neutrophile
Neutrophile
-
 Chromatin
Chromatin
-
 Nuisance
Nuisance
-
 CAF / French Avifauna Commission
CAF / French Avifauna Commission
-
 Digital rectal examination
Digital rectal examination
-
 Epidural
Epidural
-
 La Niña
La Niña
-
 Anaemia
Anaemia
-
 Granulopenia
Granulopenia
-
 Viscoelasticity
Viscoelasticity
-
 Glaucoma
Glaucoma
-
 Earth-grazing asteroid
Earth-grazing asteroid
-
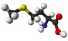 Methionine
Methionine
-
 Chiropraxis
Chiropraxis
-
 Paleocene
Paleocene
-
 Metre
Metre
-
 Therophyte
Therophyte
-
 Tilia cordata
Tilia cordata
-
 Apollo 11
Apollo 11
-
 Catchall
Catchall
-
 Tabular facies
Tabular facies
-
 Scraping
Scraping
-
 Mass storage
Mass storage
-
 MAN
MAN
-
 Island arc
Island arc
-
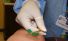 Hyperkalaemia
Hyperkalaemia
-
 Digestive system
Digestive system
-
 Immunodeficiency
Immunodeficiency
-
 Pegmatite
Pegmatite
-
 Corrosion
Corrosion
Coordination number
The coordination number is the number of atoms, molecules or ions directly bonded to a central atom in a molecular species.
Notes:
1. Examples: in methane CH4 the coordination number of carbon est 4; that of cobalt is 6 in the complex cation hexaamminecobalt Co(NH3)63+.
2. The expression is used in a different sense in the geometrical description of ionic crystals; here it is the number of nearest neighbours to the ion in question. Example: in sodium chloride the ions form a regular lattice in which each ion has a coordination number of 6.
Latest
Fill out my online form.



