-
 Appulse
Appulse
-
 Digitalin
Digitalin
-
 Dry ice
Dry ice
-
 HSR
HSR
-
 Anticancer agents
Anticancer agents
-
 Glial cells
Glial cells
-
 Heliography
Heliography
-
 Prothorax
Prothorax
-
 Semantic web
Semantic web
-
 Breast-shield
Breast-shield
-
 Large Magellanic cloud
Large Magellanic cloud
-
 Dolomite
Dolomite
-
 Pulse
Pulse
-
 Embolisation
Embolisation
-
 Increment
Increment
-
 Permafrost
Permafrost
-
 Ogg Vorbis
Ogg Vorbis
-
 Micturition
Micturition
-
 Cupid
Cupid
-
 Painted lady
Painted lady
-
 Cryoturbation
Cryoturbation
-
 Marginal basin
Marginal basin
-
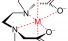 Chelation
Chelation
-
 Cladode
Cladode
-
 Topical antifungal
Topical antifungal
-
 Ribonucleotide
Ribonucleotide
-
 Firmware
Firmware
-
 Structural specificity
Structural specificity
-
 Carbohydrate
Carbohydrate
-
 Ovigerous
Ovigerous
Chalcogen
The chalcogens are the elements in column 16 (or group VI A in the old notation) of the periodic table of elements :oxygen, sulphur, selenium, tellurium and polonium.
Their outer shell has a deficit of two electrons, hence their propensity to form two bonds or a double bond, for example in the molecule H2O and the CO group (in the acidic function of an organic molecule). They also occur in the form of negative ions when they have captured the two missing electrons.
The term chalcogen means Which gives copper. Chemically, there is no direct relationship with this metal, but the main copper ores are compounds that include a chalcogen, such as copper oxide for example.
Latest
Fill out my online form.



