-
 Supplementary service
Supplementary service
-
 CD-V
CD-V
-
 Iron-bearing formation
Iron-bearing formation
-
 Lift augmentation system
Lift augmentation system
-
 Carnot cycle
Carnot cycle
-
 Microsoft System Center DPM
Microsoft System Center DPM
-
 Mumps virus
Mumps virus
-
 Semi hybrid
Semi hybrid
-
 Gliding
Gliding
-
 MNHN
MNHN
-
 Isotopologue
Isotopologue
-
 Actin
Actin
-
 Natural selection
Natural selection
-
 Rotator cuff
Rotator cuff
-
 Comet
Comet
-
 CA
CA
-
 Polylactic acid
Polylactic acid
-
 Palmitic acid
Palmitic acid
-
 Constellation of Scorpio
Constellation of Scorpio
-
 Nasal polyp
Nasal polyp
-
 Autotrophism
Autotrophism
-
 Antivenom
Antivenom
-
 Hiperlan
Hiperlan
-
 Artesian well
Artesian well
-
 Electroencephalogram
Electroencephalogram
-
 UV water steriliser
UV water steriliser
-
 Klein bottle
Klein bottle
-
 Planck constant
Planck constant
-
 Azimuth
Azimuth
-
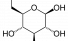 Glucose
Glucose
Cement
Cement is a hydraulic binder composed essentially of silicates and aluminates in variable proportions depending on the raw materials used:
- tricalcium silicate or C3S (3 CaO, SiO2),
- bicalcium silicate, or C2S (2 CaO, SiO2),
- tricalcium aluminate, or C3A (3 CaO,Al2O3),
- tricalcium alumino-ferrite, or C4AF (4 CaO, Al2O3, Fe3O3).
The essential raw materials are limestone and clay. They are ground and any required secondary products are added. The mixture obtained is called the raw mix and is composed of around 80% limestone and 20% clay.
In the so-called dry process, the commonest today, the raw mix is pre-heated (and dried) in a precalciner. It is then placed in a rotating cylindrical furnace, which today is always horizontal (slightly inclined). The length varies from 30 metres to 110 metres. A burner heats the interior of the furnace to between 1400 and 1500°C.
The material extracted from the furnace is called clinker and is formed of solid blocks that look like lava. This clinker is ground and 3 to 5% of calcium sulphate in the form of gypsum or anhydrite is added. There may be other additives. The result is cement. This traditional formula is for Portland cement. Other types of cement are obtained by adding various secondary ingredients.
When mixed with water, cement forms a paste that hardens as it dries. During hydration the tricalcium silicates are changed into calcium silicates and hydrates are formed. The free water disappears gradually. Cement is usually mixed with sand and aggregate to obtain concrete.
 Cement
Cement
Latest
Fill out my online form.



