-
 Chlordecone
Chlordecone
-
 Ectropion
Ectropion
-
 Tritium breeding blanket
Tritium breeding blanket
-
 Arctic fulmar
Arctic fulmar
-
 DHCP
DHCP
-
 Eruption
Eruption
-
 Fennel
Fennel
-
 Stoop
Stoop
-
 Balm
Balm
-
 Cryptocrystalline
Cryptocrystalline
-
 Tack
Tack
-
 Plain text
Plain text
-
 Reproductive success
Reproductive success
-
 Character
Character
-
 Precession constant
Precession constant
-
 Tidal
Tidal
-
 Roskosmos
Roskosmos
-
 Precambrian
Precambrian
-
 Cytokine
Cytokine
-
 Vestibule
Vestibule
-
 Dulong-Petit law
Dulong-Petit law
-
 Pit
Pit
-
 Iguanodon
Iguanodon
-
 Broad Bean
Broad Bean
-
 Soyuz-Fregat
Soyuz-Fregat
-
 Hormone therapy
Hormone therapy
-
 Benthos
Benthos
-
 Acrosome
Acrosome
-
 Blogroll
Blogroll
-
 ASIC
ASIC
Acid
There are several definitions of an acid (Arrhenius, Brönsted etc.) but the Lewis definition is the widest. This states that an acid is an electron pair acceptor (and hence possesses an empty orbital).
Conjugate acid and base
For every acid there is a conjugate base and vice versa. A base is therefore an electron pair donor. The strengths of an acid and its conjugate base are related. If an acid is strong, its conjugate base is weak, and reciprocally.
General facts about acids
An acid will colour litmus paper red and have a pH lower than 7.
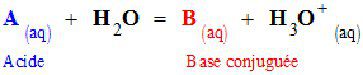
General scheme for the reaction between and acid (A) and water. The chemical equilibrium in the reaction involves the conjugate base (B) of the acid. © DR
Latest
Fill out my online form.



