-
 Cementation zone
Cementation zone
-
 Action potential
Action potential
-
 Opiate antitussive
Opiate antitussive
-
 Edaphic
Edaphic
-
 T-DMB
T-DMB
-
 Zeolite
Zeolite
-
 Ecophysiology
Ecophysiology
-
 LPG
LPG
-
 Catchall
Catchall
-
 Triazine
Triazine
-
 Diagnosis
Diagnosis
-
 Azotaemia
Azotaemia
-
 PABX
PABX
-
 Aplasia
Aplasia
-
 ESA
ESA
-
 Sedimentation
Sedimentation
-
 Herpes
Herpes
-
 Nasal polyp
Nasal polyp
-
 Myelomeningocoele
Myelomeningocoele
-
 Cyclotron
Cyclotron
-
 Enterprise
Enterprise
-
 Herschel satellite
Herschel satellite
-
 Numbering plan
Numbering plan
-
 Calamus
Calamus
-
 Levetiracetam
Levetiracetam
-
 Freshwater
Freshwater
-
 Flag
Flag
-
 Grape
Grape
-
 Nosocomial
Nosocomial
-
 Shuttering panel
Shuttering panel
Carbon monoxide
Carbon monoxide is an oxide of carbon. Its chemical formula is CO so it contains one oxygen atom and one carbon atom .
Carbon monoxide poisoning
Carbon monoxide (CO) is produced by the incomplete combustion of organic fuels (wood, butane, charcoal, petrol, diesel, natural gas, petroleum, and propane.. and is used in heaters to produce hot water or to run motors in electricity generators, for example). It is a highly toxic gas even in small amounts.
Victims of acute poisoning need immediate intensive hospital care with respiratory assistance and oxygenation in a hyperbaric chamber. These accidents can leave lifelong complications.
Concentrations of only 500 ppm (parts per million) of carbon monoxide in respired air cause severe headaches, dizziness and drowsiness indicating the onset of poisoning. Muscle failure and gradual paralysis develop when a person is exposed to concentrations of 2000 ppm, and these are followed by coma without medical assistance. Death also occurs rapidly after exposure to doses of 5000 ppm for only a few minutes. Death occurs when 66% of haemoglobin has been converted into carboxyhaemoglobin.
Studies have shown that blood in the general population in large cities contains 1 to 2% carboxyhaemoglobin. Concentrations in smokers of 4 to 10% make the heart work more in order to oxygenate the body.
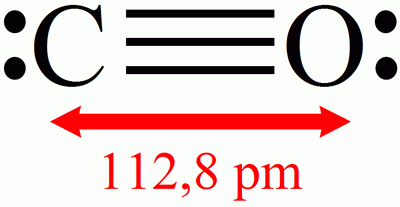
The chemical formula of carbon monoxide and interatomic distance. © DR
Latest
Fill out my online form.



